
CASOS CLÍNICOS
Inhaled anesthetic sedation,
an alternative to the scarcity of traditional sedatives: a case report
Sedación anestésica inhalada, una alternativa a la escasez de los sedantes tradicionales: reporte de un caso

CASOS CLÍNICOS
an alternative to the scarcity of traditional sedatives: a case report
Sedación anestésica inhalada, una alternativa a la escasez de los sedantes tradicionales: reporte de un caso
Recibido: 15-06-2022
Aceptado: 07-09-2022
Publicado: 30-12-2022
Revista MetroCiencia
Volumen 30, Número 4, 2022
ISSNp: 1390-2989 ISSNe: 2737-6303
Editorial Hospital Metropolitano
Jorge Luis Vélez-Páez1,2*, Fernando Esteban Jara1, Esteban Ochoa1, Verónica Guerrero1, Estefanía Irigoyen1, Hernán Quintero1, Eduardo Vásconez3, Pablo A. Santillán4, Esteban Ortíz-Prado3
ABSTRACT
El síndrome de Apert (SA) es una patología poco frecuente, caracterizada por malformaciones faciales, craneosinostosis primaria y malformaciones simétricas de las manos y los pies, afecta igual a hombres y mujeres. Se debe a dos mutaciones en el receptor del factor de crecimiento de fibroblastos en el cromosoma 10. La anatomía patológica de la mano incluye sindactilia compleja, con fusión ósea que afecta segundo, tercer y cuarto dedo, además incluye pulgar corto con clinodactilia radial asociado a una duplicación o inserción anormal del músculo abductor pollicis brevis. Se utiliza la clasificación establecida por Upton en 1991, quien clasificó la sindactilia del SA según la severidad en tipo I mano en paleta, tipo II mano en manopla y tipo III mano en capullo de rosa y debe ser usada para guiar manejo quirúrgico. En deformidades donde el pulgar tiene sindactilia simple incompleta será suficiente la profundización de primer espacio interdigital. En caso de sindactilia compleja o simple completa, se realiza corrección del primer dedo.
Keywords: AnaConDa, volatile anesthetic, COVID-19.
RESUMEN
Apert syndrome (AS) is a rare pathology characterized by facial malformations, primary craniosynostosis and symmetrical malformations of the hands and feet, affecting males and females equally. It is due to two mutations in the fibroblast growth factor receptor on chromosome 10. The pathologic anatomy of the hand includes complex syndactylia, with bony fusion affecting the second, third and fourth fingers, and also includes short thumb with radial clinodactylia associated with a duplication or abnormal insertion of the abductor pollicis brevis muscle. The classification established by Upton in 1991, who classified HS syndactylia according to severity into type I paddle hand, type II mitten hand and type III rosebud hand is used to guide surgical management. In deformities where the thumb has incomplete simple syndactylia, deepening of the first interdigital space will be sufficient. In case of complex or complete simple syndactylia, correction of the first finger is performed.
Palabras claves: AnaConDa, anestésico volátil, COVID-19.
Jorge Luis Vélez-Páez
 https://orcid.org/0000-0002-6956-4475
https://orcid.org/0000-0002-6956-4475
Fernando Esteban Jara
 https://orcid.org/0000-0003-2132-7187
https://orcid.org/0000-0003-2132-7187
Esteban Ochoa
 https://orcid.org/0000-0001-6981-553X
https://orcid.org/0000-0001-6981-553X
Verónica Guerreo
 https://orcid.org/0000-0003-3762-1890
https://orcid.org/0000-0003-3762-1890
Estefanía Irigoyen
 https://orcid.org/0000-0002-4904-1124
https://orcid.org/0000-0002-4904-1124
Hernán Quintero
 https://orcid.org/0000-0001-6929-9774
https://orcid.org/0000-0001-6929-9774
Eduardo Vásconez
 https://orcid.org/0000-0003-4573-6217
https://orcid.org/0000-0003-4573-6217
Pablo A. Santillán
 https://orcid.org/0000-0001-6240-7461
https://orcid.org/0000-0001-6240-7461
Esteban Ortíz-Prado
 https://orcid.org/0000-0002-1895-7498
https://orcid.org/0000-0002-1895-7498
 |
Usted es libre de:Compartir — copiar y redistribuir el material en cualquier medio o formato. |
*Correspondencia: jlvelez@uce.edu.ec
INTRODUCCIÓN
Sedation in critically ill patients undergoing invasive mechanical ventilation reduces anxiety and agitation, facilitates an adequate ventilatory coupling, and allows invasive procedures. Many drugs are used to reach this end, among them midazolam and propofol; however, these drugs have pharmacological characteristics that keep them from being an ideal sedative since they must have a rapid onset and displacement, not alter hemodynamics, avoid their accumulation with prolonged use, and be eliminated relatively quickly when suspended1,2.
COVID-19 can manifest as severe pneumonia that progresses to acute respiratory distress syndrome (ARDS), which in many cases requires mechanical ventilation and deep sedation. The exponentially increasing number of critical cases have saturated even the most robust health systems and have depleted the world's pharmacological reserves of sedative drugs, leaving us, on several occasions, without therapeutic alternatives3,4. Volatile anesthetics (sevoflurane, isoflurane) have constituted an important part of general anesthesia in operating rooms; however, they have been used very little in Intensive Care Units. Considering that the pathophysiology of COVID-19 is directly related to a strong pro-inflammatory immune response, it has been seen that sedation with volatile inhalation agents can reduce the severity and progression of the disease and become an alternative by avoiding many of the side effects that are associated with the use of intravenous sedatives3-5. In 2005, the AnaConDa device, which stands for Anesthetic Conserving Device (ACD) (Figure 1), was marketed allowing the administration of inhalational drugs with standard ventilators in critical care units. It consists of a modified heat and humidity exchanger filter, which, inserted in the patient's ventilatory circuit, allows the administration of halogenated agents through a vaporization chamber connected to a syringe with the liquid anesthetic1,6,7.
Some of the advantages of inhalation sedation over intravenous sedation are8:
Inhalation sedation use has several indications and contraindications9, which are summarized in Table 1.

The case of a patient with a confirmed diagnosis of COVID-19 is presented, who develops severe ARDS with subsequent need for deep sedation, refractory to traditional drugs, with a good response to a volatile anesthetic (Sevofluorane) with use of the AnaConDa system.
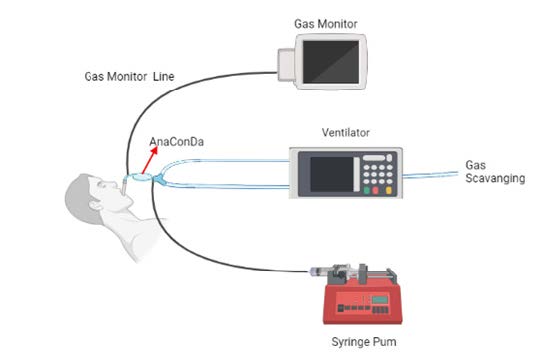
CLINICAL CASE
50-year-old Hispanic male, without significant comorbidity. He presented after 14 days of headache, rhinorrhea, and general malaise progressing to dyspnea. He was admitted with tachycardia of 130 beats per minute, tachypnea of 40 breaths per minute, use of accessory muscles, oxygen saturation of 74% in ambient air and PaO2/FiO2 ratio of 84. Non-invasive mechanical ventilation was poorly tolerated, therefore invasive mechanical ventilatory support was started in the prone position. The chest radiograph showed diffuse bilateral interstitial alveoli infiltrates (Figure 2). He was admitted to the ICU, under the effects of sedoanalgesia and a muscle relaxant based on remifentanil at 14 mcg/kg/h, propofol at 5 mg/kg/h, and rocuronium bromide at 0.12 mg/kg/h, respectively; the prone position was maintained. He presented shock with a mean arterial pressure of 55 mmHg and a heart rate of 48 beats per minute, which warranted management with norepinephrine-type vasopressor support at 0.08 mcg/kg/min, classifying it as a pharmacological distributive shock.
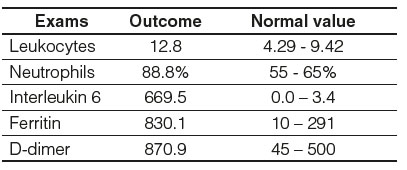
In the laboratory analysis as relevant findings, leukocytes of 12.80 (NV: 4.29 - 9.42), neutrophils 88.8% (NV: 55 - 65%), interleukin 6 (IL-6) 669.5 (NV: 0.0 - 3.4), ferritin were found 830.1 (NV: 10-291) and D-dimer 870.9 (NV: 45-500) (Table 2).
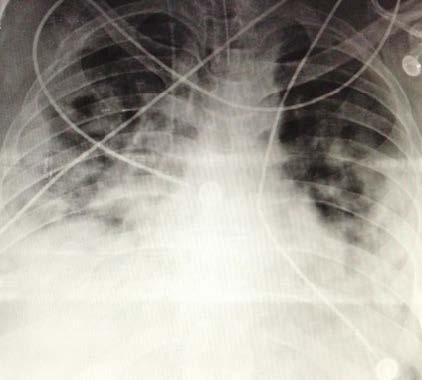
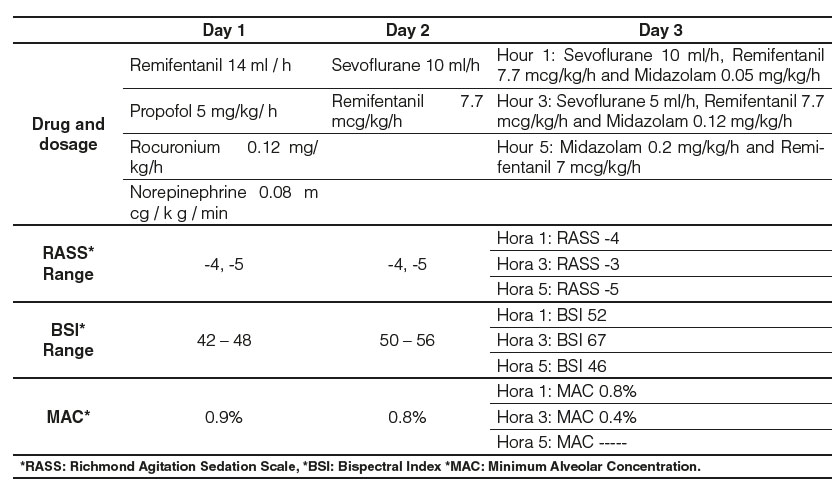
In the ICU, he developed tolerance to the sedative effect of propofol, for which the infusion was withdrawn and sevoflurane was started using AnaConDa. Monitoring was carried out employing the bispectral index (BIS) with objectives of 40 to 60 and the dose was titrated according to the patient's response. The initial dose on the first day of hospitalization was 14 ml/h, obtaining a BSI of 42 and a minimum alveolar concentration (MAC) of 0.9%, achieving goals of deep sedation RASS -4 or -5. On day 2, the infusion of the muscle relaxant was suspended and remifentanil infusion was started at a dose of 7.7 mcg/kg/h, thereby reducing the dose of sevoflurane, maintaining a RASS -5 and BIS 50. For day 3 and after meeting oxygenation targets, the patient was placed in the supine position and the strategy was changed to intravenous sedation (midazolam), which was progressively increased to a dose of 0.2 mg/kg/h with a concomitant decrease in sevoflurane until his definitive retirement (Table 3).
During his hospital stay, the patient did not develop delirium or renal failure and the vasopressor dose was reduced until it was withdrawn within 24 hours, maintaining hemodynamic stability at all times
Successful extubation was achieved 48 hours later and subsequent discharge to the hospital ward with oxygen support at low flow through a nasal cannula (2 L/min).
DISCUSSION
The global shortage of intravenous sedatives and neuromuscular blocking agents (benzodiazepines, opioids, propofol), has become a problem for the provision of care of critical patients during the COVID-19 pandemic¹. Evidence indicates that inhaled agents such as isoflurane and sevoflurane offer sedation, muscle relaxation, and maybe beneficial at low doses due to their lung clearance, anti-inflammatory, bronchodilator effects in the airways, lungs, and vascular beds, also taking into account They do not have a great analgesic effect and are therefore co-administered with intravenous opioids, in addition, they shift the hemoglobin dissociation curve to the right, increase the release of oxygen from hemoglobin to the tissues, and improve tissue oxygenation1,3,10. Volatile agents reduce macrophage levels in the bronchial alveolar fluid, therefore, they also reduce the release of TNF, IL-6, IL-1, monocyte chemotactic protein-1 (MCP-1), decrease neutrophilic adhesion and production of reactive oxygen species giving rise to a powerful inhibitory effect of pro-inflammatory cytokines and a stimulating effect of anti-inflammatory cytokines, in the same way, it significantly inhibits platelet aggregation3,5.
Prolonged use of volatile agents demonstrated good safety with hemodynamic stability, absence of liver and kidney toxicity, and less delirium, compared to classic intravenous agents. In addition, it facilitates synchrony with the ventilator, prone strategy, and extracorporeal oxygenation therapy (ECMO) with deeper levels of sedation. Volatile anesthetics, due to their low metabolism and solubility in blood, avoid tolerance or addiction phenomena, allowing a faster awakening compared to conventional intravenous sedatives such as propofol or midazolam, they shorten the awakening time by 80 minutes and extubation by 196 minutes. Adverse effects are very rare but can include nephrogenic diabetes insipidus, malignant hyperthermia, allergy, and hepatitis3,10,11. Several authors have recommended that it is important to maintain deep sedation for patients with COVID-19 who are admitted to mechanical ventilation to minimize aerosolization12, however, the requirements are high, currently, sedative drugs are in short supply. alternatively, inhalation sedation with its previously described ones, the study carried out by Flinspach et al.4 highlighted that there was no liver and kidney toxicity. The study by Jabaudon et al.13 who compared sevoflurane vs midazolam for sedation in ARDS showed that patients who used inhaled sevoflurane improved oxygenation and decreased levels of endothelial markers compared to sedation with midazolam. However, concerning COVID-19, at the same time that it was shown to decrease the inflammatory response and platelet aggregation, it was also associated with adverse effects such as immune dysfunction in cases of sepsis and diabetes insipidus10,11.
Currently, the management of sedation in COVID-19 patients is based on standard guidelines for intensive care and on the experience of treating patients with "classic" ARDS. There are insufficient studies on the impact of volatile sedation on the pathophysiology of the disease. COVID-19, but it has been seen that it could be beneficial3. In the present clinical case, the administration of sevoflurane in continuous infusion into the inhalation precipitate system (AnaConDa) was indicated due to tolerance to traditional sedatives, with this it was evidenced that our patient presented better ventilatory coupling in the prone position, even without requiring treatment with neuromuscular relaxants, in addition to achieving greater hemodynamic stability and no side effects; delirium was not evidenced and there was no need to add a second sedative drug to achieve the stated sedation goals14. We consider this system useful especially when there is difficulty in adapting patients to the mechanical ventilator and in those cases in which very high doses of sedation are required. Their pharmacokinetics and pharmacodynamics make them an attractive option for sedation in the ICU, probably improving the time of extubation and discharge from intensive care. For all these reasons, the use of this sedation modality is proposed, being a valid and effective option.
CONCLUSIONS
Sedation in critically ill patients is a crucial issue, the global shortage of intravenous sedatives and neuromuscular blocking agents has become a problem for the provision of care of critical patients during the COVID-19 pandemic. Evidence indicates that the Inhaled agents such as isoflurane and sevoflurane offer sedation, muscle relaxation, and maybe beneficial at low doses. However, there are still no large studies that conclusively demonstrate the benefit of these drugs in important outcomes such as mortality. At the moment, sedation with volatile anesthetics can be considered an appropriate alternative in specific patients, but it is costly compared to traditional sedatives.
Contribución de los autores
Los autores declararon no tener ningún conflicto de interés personal, financiero, intelectual, económico y de interés corporativo con el Hospital Metropolitano y los miembros de la revista MetroCiencia.